MASS SPECTROMETRY

Biotechnology is the study of biological materials to create new technology.
These biological materials are normally very large molecules,
such as proteins,
polymers
and nucleotides.
In fact,
they are called macromolecules
because they are made of thousands of atoms.
It can be very difficult to identify what is inside a mixture of macromolecules.
The best way is time-of-flight mass spectrometry
(TOF-MS).
The idea behind this type of spectrometry is simple.
First,
the molecules are ionised.
This means that some electrons are removed from them
and they become positively charged.
An electric field then accelerates the new positive ions
and they reach a detector after different amounts of time,
according to their mass.
We ionise molecules before the experiment
because molecules that do not have an electric charge
do not interact with an electric field.
This means that neutral molecules
will not be accelerated.
Different spectrometers
ionise molecules in different ways.
A very common way of ionising molecules,
especially big molecules,
is MALDI
(Matrix-Assisted Laser Desorption/Ionisation).
In this technique,
the sample forms crystals in a matrix with another material.
Next,
a laser hits the crystal matrix,
making the molecules a gas and ionising them
(this process is called desorption/ionisation).
An electric field accelerates the charged, gaseous molecules in a vacuum chamber.
Finally,
they drift with no electric or magnetic field
in a time-of-flight tube
until they arrive at the detector,
as you can see in the following scheme.
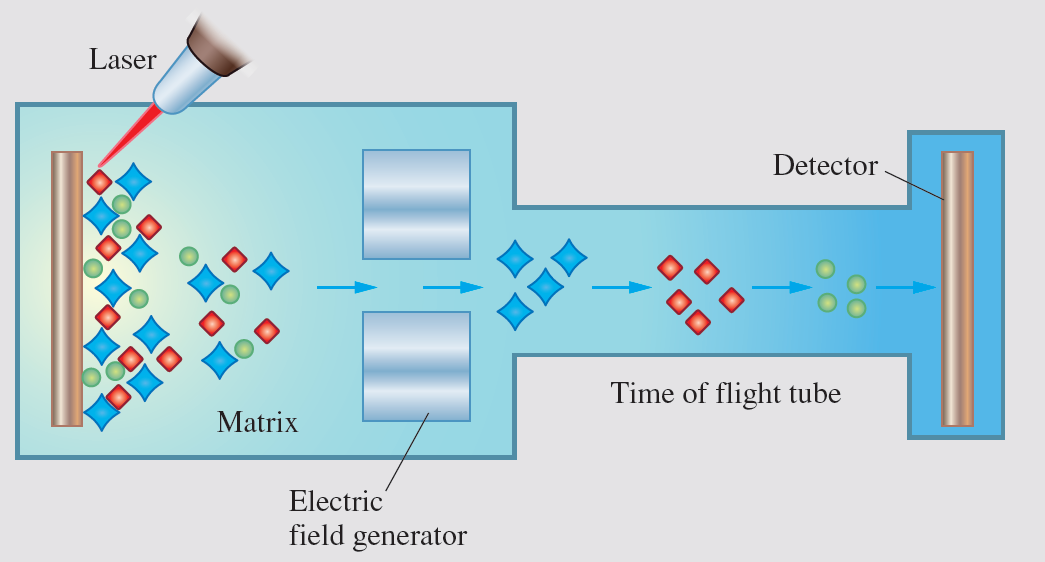
Diagram of a MALDI-TOF Mass Spectrometer.
Newtonian dynamics
can help us to understand the movement of the molecules.
The ionised molecules are accelerated in the vacuum chamber
which has a length of l.
The velocity v that they reach
depends on acceleration a
according to the following equation:
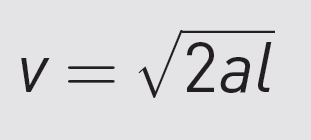
Newton’s second law gives us an equation for acceleration:
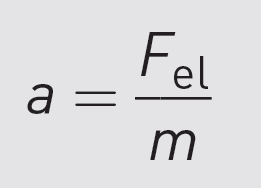
where m is the mass of the molecule.
If we substitute for a in the velocity equation,
we get:
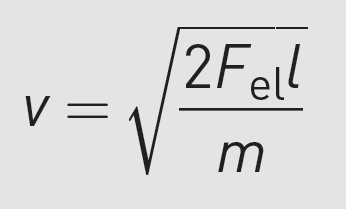
This is the velocity that the molecules have in the drift tube
(where their motion is uniform).
You can see that velocity is inversely proportional to the square root of mass.
This means
that the heaviest molecules are the slowest
and lighter molecules move more quickly.
This is how a time-of-flight mass spectrometer can understand the mass of a molecule.
If d is the length of the drift tube,
we can calculate how long it takes for the molecule to arrive at the detector:
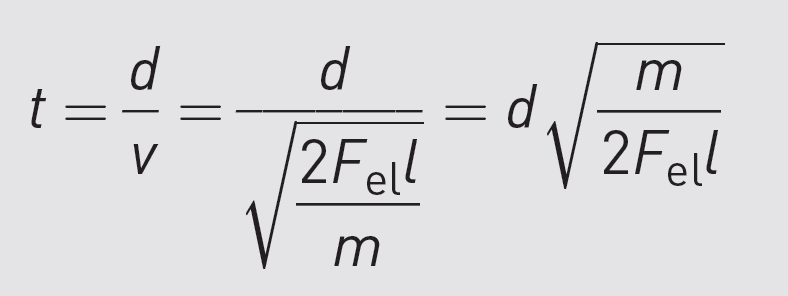
From this time t,
you can calculate mass.
From the mass,
you can identify a molecule,
which is exactly what we want to do!
The spectrometer converts all this information about mass into a graphic
which we call a mass spectrum.
This graph shows the mass on one axis
and the relative abundance of that molecule on the other axis.
You can find an example of a mass spectrum below.
Mass spectra help us to identify molecules
because each molecule has a unique spectrum of masses.
We compare the spectrum of our sample
with the spectra of many molecules
to see which spectrum matches.
A MALDI-TOF mass spectrum of glycans from Ebola glycoprotein.
VOCABULARY
detector = rilevatore
to drift = andare alla deriva
to ionise = ionizzare
mass spectrometry = spettrometria di massa
to match = abbinare
mixture = miscuglio
relative abundance = abbondanza relativa
square root = radice quadrata
time-of-flight mass spectrometry = spettrometria di massa a tempo di volo
time-of-flight tube = tubo di deriva
vacuum chamber = camera a vuoto

