The Second Law of Thermodynamics
We all know that if a wheel is heated up, it will not start turning around all by itself – though this would be allowed by energy conservation. It’s not impossible but highly, indeed highly improbable. On the other hand, a steam engine does convert heat into work. The important question “to what extent can heat be converted to work?” is answered by the second law of thermodynamics, undoubtedly the most famous of the three.
This law featured in the famous essay The two cultures by C.P. Snow, published in 1956, in which Snow publicly criticised the scientific illiteracy of well educated people by noting the fact that virtually everybody knows a play by Shakespeare but virtually nobody knows what the – equally important – second law of thermodynamics is about!
The Kelvin formulation of this law states that there is no thermodynamic process whose sole effect is to extract heat from a reservoir and to convert it entirely into work. This can be paraphrased by saying that an ideal engine cannot exist as a matter of principle. This has to be contrasted with a real engine, which is a machine that goes through a cycle of thermodynamic states and does convert heat into work, but at the same time has to deliver part of the extracted heat to the environment.
The second law involves the notion of entropy, which is a variable state like temperature, but more subtle because one cannot measure it directly. In terms of entropy, the second law of thermodynamics states that for any process taking place in a closed system, the entropy cannot decrease.
Entropy is a measure for the disorder of a system. This definition can be made rigorous if one studies the statistical connection with the accessible states of the system at the microscopic level. In the field of statistical mechanics, one can establish that in a closed system which is not in equilibrium, entropy increases until it reaches a maximum at equilibrium. This is basically a consequence of the trend “to go from a less probable to a more probable state”.
[Adapted from S. Bais, The Equations. Icons of Knowledge, Harvard University Press, 2005]

William Thomson, 1st Baron Kelvin (1824-1907).
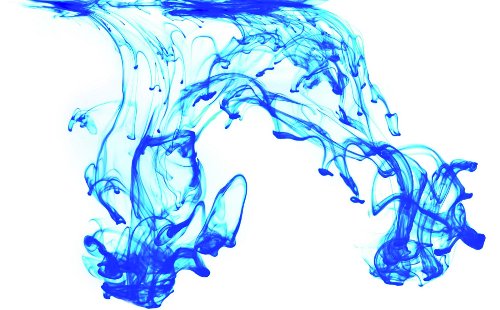
If a drop of ink is released in a container filled with water, the ink molecules will scatter around and spread out in the water until they have reached the equilibrium situation where the molecules are distributed uniformly throughout the container. Clearly, the opposite process will not happen, certainly not spontaneously.
Minidictionary
Disorder = Disordine
Entropy = Entropia
Environment = Ambiente
Reservoir = Sorgente
Steam engine = Macchina a vapore
Thermodynamics = Termodinamica

